Additional Information
| Product Name: | APPBP1/6xHis-UBA3 | ||
|---|---|---|---|
| Also Known As: | APPBP1 (NAE1;HPP1; ula-1; A-116A10.1); UBA3 (UBE1C; hUBA3; MGC22384; DKFZp566J164) | ||
| Catalog No.: | B1400 | ||
| Size: | 25 µg | ||
| Molecular Weight: | APPBP1 (60.2 kDa), UBA (51.8 kDa) | ||
| Species: | Human | ||
| Source: | Bacterial recombinant | ||
| Stock: | 20 mM Tris, 150 mM NaCl, 2 mM βME, 10% Glycerol | ||
| Concentration: | See tube label | ||
| Quality Assurance: | ~95% by SDS-PAGE, see datasheet for gel image | ||
| Storage: | Store at -80°C; avoid multiple freeze-thaw cycles | ||
| PDF Data Sheet: | PDF Datasheet, MSDS | ||
| NCBI RefSeq: | NM_003905 (APPBP1); NM_003968 (UBA3) | ||
| Image(s): | (Click image to enlarge)
|
||
| Shipping Method: | Dry ice shipping | ||
| References: | 1. Whitby FG, et al. (1998) J Biol Chem 273(52), 34983 – 34991. 2. Osaka F, et al. (1998) Genes Dev 12(15), 2263 – 2268. 3. Walden H, et al. (2003) Mol Cell 12(6), 1427 – 1437. 4. Schulman BA, et al. (2009) Nat Rev Mol Biol 10(5), 319 – 331. |
Details
APPBP1/UBA3 is the heterodimeric E1 enzyme that conjugates Nedd8 to protein substrates. Nedd8 is conjugated onto the catalytic cysteine residue of UBA3 by formation of a thioester bond in an ATP-dependent reaction. The activated Nedd8 is then passed on to an E2 (Ubc12) to be finally attached to a substrate. Nedd8 is mainly conjugated on Cullins to increase the activity of the Cullin – RING E3 Ub ligases.
Images:
(Click image to enlarge)
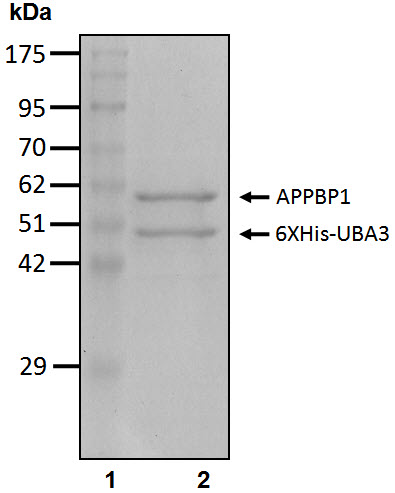
|
|
Coomassie-stained SDS-PAGE Lane 1: Molecular weight markers Lane 2: 5 µg purified APPBP1/6xHis-UBA3 |